Which of the Following Is an Effective Model for Understanding the Role of Symptoms in the Family?
Introduction
The current pandemic of Coronavirus Disease 2019 (COVID-xix), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has undergone an observed exponential increase of cases that has overrun hospitals across the world (1). Many people have mild forms of the disease and are advised not to get to the hospital or to seek a diagnostic exam considering they tin recover at home. A large number of others are asymptomatic (2). Infected individuals are highly contagious and can transmit the disease fifty-fifty if they are asymptomatic, and this fact furthers the need to isolate and test oft (2). In addition, COVID-19 is two to three times more contagious than influenza (3). Due to these characteristics, outbreaks of COVID-nineteen occur in clusters (4). Identifying COVID-19 early could reduce the number and size of clusters, but early symptoms are not well-divers. The Center for Disease Control and Prevention (CDC) in the United states and the World Wellness Organization (WHO) currently advise the public to call their dr. if they believe they have been exposed to COVID-nineteen or exhibit fever and coughing (five). However, fever and cough are associated with other respiratory diseases such as flu (6–8). Influenza, with an estimated number of symptomatic cases in the millions annually in the U.Due south. lone (9), as well is commonly associated with fever and cough (half-dozen). Similarly to COVID-xix, the Eye East Respiratory Syndrome (MERS) and the Severe Acute Respiratory Syndrome (SARS) are respiratory illnesses contracted from coronaviruses called the MERS-Related Coronavirus (MERS-CoV) and SARS-Related Coronavirus (SARS-CoV), respectively (7). The symptoms of these diseases also overlap with COVID-xix. The capacity to discern differences in these mutual symptoms, such as club of occurrence and likely offset symptoms, would aid in early recognition. If health care workers recorded and published clinically-observed and/or patient-reported sequences of symptoms, the reported data could be evaluated as an additional tool for early on recognition of COVID-nineteen to increase cocky-surveillance and reduce spread. If such a widespread clinical practice had been instituted in the past, possibly local outbreaks of influenzas, coronaviruses, and other diseases might have been contained earlier becoming pandemics.
To this finish, we assumed that symptoms and their orders are independent variables and created a model that approximates the probability of symptoms occurring in specific orders using available, non-ordered patient information. The use of these assumptions and information was necessary given the lack of ordered data. To do this, we applied a Markov Process to determine the order of occurrence of common symptoms of respiratory diseases. We have previously used a Markov Concatenation to predict cancer metastasis location (10–14). A Markov Process is divers as a stochastic sequence of events in which the likelihood of the next state only depends on the current state rather than past or time to come states (fifteen). In this case, we divers each state to be the specific symptoms that a patient has experienced, and each transition is merely dependent on these symptoms. Every bit a effect, nosotros can determine the likelihood of each symptom stepwise using a Markov Process. Nosotros defined the state probability of a node as the frequency that a patient has a item combination of symptoms divided by the total number of patients that exhibit the aforementioned number of symptoms. The transition probability betwixt two states is defined equally the likelihood of acquiring a single specific symptom divided past the likelihood of acquiring all possible next symptoms. We and then practical a greedy algorithmic approach using the transition probabilities to calculate the probability of all possible orders toward determining the most and least probable orders of symptoms.
In this report, nosotros first defined this specific application of a Markov Process practical to a graded partially ordered prepare (poset), which we refer to equally the Stochastic Progression Model. In this case, our graded poset represents all possible combinations of symptoms and all possible orders of symptom occurrence. It is graded because the possible combinations of symptoms are ranked by the number of symptoms that they each represent. For example, the symptom combination of fever and cough has the same rank every bit the combination of cough and diarrhea. We institute that the Stochastic Progression Model for adults that are symptomatic indicates that there may exist an order of discernible symptoms in COVID-19, but the order of symptoms seems to be independent of severity of the instance on admission. From there, nosotros compared the most likely society of symptoms in other respiratory diseases to COVID-19. To expand on our results, we analyzed a larger set up of symptoms that are common to all respiratory diseases studied here and sought to decipher further distinctions.
Materials and Methods
Data Collection
Patient data from this study was nerveless from various reports in literature on the frequencies of symptoms in COVID-19, influenza, MERS, and SARS (Supplemental Tables 1, 2). Each dataset was used either to approximate order of symptoms, to confirm our results, or to clarify first symptoms in COVID-xix or flu. For all of these applications, we used the reported patient data to simulate patients with various combinations of symptoms experienced and then applied the simulated data to perform the analyses.
The main dataset of COVID-xix patients of the Earth Health Organisation, containing 55,924 confirmed cases, was obtained through review of national and local governmental reports and observations fabricated during visits to areas with infected individuals in Red china that occurred from February 16 to 24, 2020 (8). A confirmation dataset of COVID-19 patients, containing ane,099 confirmed cases, was obtained past the China Medical Treatment Practiced Grouping for COVID-xix from medical records and other compiled information of hospitalized patients and outpatients that were diagnosed with COVID-19. This information was reported to the National Health Committee of China from Dec 11, 2019 to January 29, 2020 (16). For both COVID-19 datasets, myalgia was reported as myalgia or arthralgia. We assumed that almost patients with myalgia likewise had arthralgia, and therefore nosotros used the frequency of myalgia or arthralgia as a frequency for myalgia when simulating data. The flu dataset, containing ii,470 confirmed cases, was collected by researchers at the University of Michigan from a retrospective pooled analysis of more often than not unvaccinated patients participating in stage two and 3 clinical trials that were conducted in Due north America, Europe, and the Southern Hemisphere from 1994 to 1998 (6). This group of patients has a hateful age of 35 and each exhibited multiple symptoms. Vomiting and diarrhea were not reported in this influenza dataset, but they are common among respiratory disease. Although adult patients at times may experience vomiting and diarrhea when infected with influenza, these symptoms are rare (17). Therefore, nosotros approximate the frequency of these symptoms as 0.010 in this example. The datasets representing symptom frequency in MERS, containing 245 patients, and SARS, containing 357 patients, were collected on admission and were reported as clinical information from physicians, Dr. Yin, at the Beijing Chao-Yang Hospital and Dr. Wunderink, at the Northwestern University Feinberg School of Medicine (7). The patients included in these datasets varied in age and pre-existing conditions. In the cases of SARS, the patients tended to be younger and have fewer pre-existing weather condition than in the cases of MERS.
Nosotros used initial frequency data of MERS and SARS to further ascertain early symptoms of illness. The MERS initial symptom frequency dataset, containing 45 confirmed cases, was nerveless from electronic medical records at the Samsung Medical Centre in Seoul, Southward Korea that contained onset symptom information virtually patients in the 2015 Korean MERS outbreak (Supplemental Table 3) (18). The SARS initial symptom frequency dataset, containing 144 confirmed cases, was collected from hospital records including data of early on symptoms in patients dating from March 7 to Apr ten, 2003 during an outbreak in the greater Toronto area (Supplemental Table 4) (19).
Lastly, ii additional datasets were collected to determine the utility of using first symptoms every bit early indicators of COVID-xix and influenza. The COVID-xix dataset used, containing 138 patients, was independent of all prior COVID-19 datasets. This data was obtained from electronic medical records of patients admitted to the Zhongnan Hospital of Wuhan University from January 1 to 28, 2020 (20). The symptom data was collected at onset of disease and all patients experienced pneumonia due to COVID-19. In this dataset, nausea and vomiting were reported separately for COVID-xix. We causeless that most patients who experience vomiting, which is reported with a frequency of 0.036, too experience nausea, which is reported with a frequency of 0.101, and therefore to simulate the data, we divers the frequency for nausea/airsickness as 0.101. The influenza dataset used reported 20 confirmed cases of influenza and 400 confirmed negative cases of influenza and is independent from any other influenza dataset nosotros used (21). The symptom information was collected through questionnaires and observations by medical professionals during the influenza seasons of 2006 and 2007 of infected patients admitted at the Department of Internal Medicine and Infectious Diseases and the Department of Pulmonology at the University Medical Eye Utrecht. Like the other influenza dataset described in a higher place, vomiting and diarrhea were not reported in this dataset. So, we again causeless the frequency of these symptoms to be 0.010 (17). Because this study was conducted in 2006 and 2007, prior to the COVID-19 outbreak, we assumed these patients were negative for COVID-19 likewise. Then, this 400-patient grouping was used as the dataset that represents individuals negative for both COVID-19 and influenza (Supplemental Table five).
Simulating Symptom Progression From Patient Data
The Stochastic Progression Model was congenital in R under version three.5.2 and was illustrated by using the hasse function in the hasseDiagram_0.ane.three library (code bachelor online: https://github.com/j-larsen/Stochastic_Progression_of_COVID-19_Symptoms) (22, 23). Each respiratory disease report was represented past a corresponding data frame, with columns as symptoms, ane row as the frequency of the symptoms observed in the report, and the other row every bit the frequency multiplied past 1,000. The multiple of the frequency is divers every bit the frequency count, which represents the probability of a symptom in a theoretical sample size of 1,000 simulated patients. Additionally, the land of an private is displayed through a character array of ones and zeros, where ones represent the presence of a symptom and zeroes represent its absence. This process of simulating a symptom is analogous to a jar of marbles of either 2 colors. The probability of pulling one color of marble (i.e., a specific symptom) is illustrated by the frequency count because the total number of marbles in the jar is 1,000 and the frequency count for each is the number of the specific color of marbles in the jar.
We so simulated data of 500,000 patients, past randomly selecting if a patient has or does not have a symptom using the procedure described above and storing that information in a data frame that represents patients equally rows and symptoms as columns. Nosotros assumed the occurrence of symptoms are random and independent. Because these assumptions, nosotros built the character arrays by applying the jar of marbles method for each false patient. The method repeats for each patient and involves pulling a marble from a series of jars representing each symptom. The data from each randomly pulled marble is stored in the corresponding cell of the character array in the correct column representing the symptom and the row representing the simulated patient. This process is repeated for all 500,000 simulated patients for all symptoms.
Building the Stochastic Progression Model
The Stochastic Progression Model is illustrated equally a directed acyclic graph with nodes, representing the power set up of Boolean vectors. The ability sets of Boolean vectors each represent a possible state of a patient by noting the absence or presence of specific symptoms. The edges, which illustrate the transition from ane state to some other, were selected specifically using key definitions and assumptions to create a poset. Nosotros defined united states of america at the nodes every bit symptoms that a patient has experienced up until this indicate. We created and directed edges from states with fewer symptoms to more starting at the minimum set up of a Boolean vector of all zeros, which indicates a person with no symptoms. Beginning, we assume that each symptom occurs one at a time, fifty-fifty if the difference in time is infinitesimal. With this supposition, a node can simply exist directed to other nodes that denote the aforementioned set of symptoms plus one boosted symptom. Second, nosotros assume that if a patient does non digress and does not die, they volition eventually acquire all symptoms reaching the maximum ready of a Boolean vector, which represents a patient that has exhibited all symptoms. Applying these assumptions to form the directed acyclic graph creates a Hasse Diagram of a graded poset that follows a Markov Process birthday comprising the Stochastic Progression Model.
Calculating Country and Transition Probabilities
The nodes in the Hasse Diagram represent states of a patient by indicating the specific symptoms exhibited, and the edges represent transitions between these states. Therefore, we next needed to employ state probabilities to each node and transition probabilities to the directed edges. First, we labeled each simulated patient by summing the respective Boolean vector to find the number of symptoms for each patient. So, to go the state probability of each node, we divided the number of simulated patients that are represented by the current Boolean vector by the total number of patients who have the aforementioned number of symptoms. To guess the transition probability betwixt two nodes (originating and terminating), we divided the number of simulated patients that are represented by the terminating node by the number of simulated patients that are represented past nodes characterized by the same number of symptoms as the terminating node, including the terminating node. The error of each node is determined past the sum of the products of the transition probabilities leading to that node subtracted from the state probability of the node. So, the fault of each implementation of the model was defined as the fault of the node with the highest accented value of error (Supplemental Figures 2–13). The transition probabilities signify the likelihoods of transitions from ane node to another, and the aggregates of the transition probabilities in a sequence correspond the likelihoods of the paths. These paths illustrate the club of symptoms when infected with a respiratory disease by observing the stepwise addition of symptoms when traversing down nodes in the path. The most and to the lowest degree probable paths were determined using a greedy algorithmic arroyo. This approach consists of selecting local maximum or minimum edges stepwise, which results in a almost and to the lowest degree likely path, respectively. If the maximum (or minimum) transition probability from a specific node was within error of other transition probabilities of edges from the aforementioned originating node, nosotros grouped the terminating nodes when finding the most (or to the lowest degree) likely path. In these cases, nosotros could not distinguish a difference in likelihood betwixt these specific transitions. The paths create a possible order of symptoms via the poset, each having a specific likelihood of occurrence.
Results
A Possible Gild of Discernible Symptoms in COVID-nineteen
The WHO-China Joint Report from February sixteen to 24, 2020 includes rates of symptom occurrence at presentation from 55,924 confirmed cases of COVID-19 (viii). We identified symptoms that were easily discernible or objective (i.e., fever, cough, diarrhea, and nausea/vomiting) in comparison to other reported symptoms, such as inflammations of blood vessel epithelia (24), neurological furnishings (25), and rash-similar symptoms (26). These symptoms are as well common in other respiratory diseases. Thus, nosotros chose to implement these 4 symptoms in the Stochastic Progression Model (Supplemental Table 1). To confirm the validity of the model, we first determined the possible sequences of symptom occurrence when the probabilities are uniformly random for each symptom. In addition to all possible orders of occurrence of the four symptoms, the diagram displays the most and to the lowest degree probable paths of the iv symptoms, depicted by ruby-red lines and blue lines, respectively (Figures 1A,B). The about and least likely paths describe the most and to the lowest degree likely series of symptoms that a random infected person from the population in the dataset may experience. In this case, each possible path is equally likely, with no path having any higher probability than whatever other.
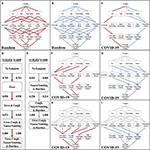
Figure i. Development of the stochastic progression model for COVID-nineteen. (A) The almost likely paths (ruby-red) in the Hasse Diagram for symptoms with random likelihoods of occurring. (B) The least probable paths (blue) in the Hasse Diagram for symptoms with random likelihoods of occurring. (C) The most likely (crimson) and least probable (bluish) paths in the Hasse Diagram for symptoms in COVID-19. (D) The nigh likely social club of symptoms in COVID-nineteen based on our Stochastic Progression Model determined from transition probabilities presented here. (Due east) The to the lowest degree likely club of symptoms in COVID-19 based on our Stochastic Progression Model determined from transition probabilities presented here. (F) Hasse Diagram of the most likely paths (crimson) after traveling whatsoever forced path (gray) of patients with 1 symptom. (K) Hasse Diagram of the least probable paths (blue) later traveling whatsoever forced path (greyness) of patients with one symptom. (H) Hasse Diagram of the near likely paths (cherry-red) after traveling whatsoever forced path (gray) of patients with two symptoms. (I) Hasse Diagram of the to the lowest degree likely paths (bluish) afterwards traveling whatsoever forced path (gray) of patients with two symptoms.
We then created another implementation of the Stochastic Progression Model and utilized the data in the WHO-China Joint Report (COVID-xix with North = 55,924) (viii). With this implementation, we determined the most and least likely paths (Figure 1C). In this instance, a person infected with COVID-xix is most likely to feel symptoms in the social club of fever, cough, nausea/vomiting, and then diarrhea (Figure 1D). The least probable path starts at diarrhea and nausea/airsickness and is followed by coughing, and finally fever (Figure 1E). We confirmed these results with a smaller dataset (COVID-xix with N = 1,099) (Figures 1D,E, and Supplemental Figure 1) (sixteen). The likelihoods of transitioning to fever, 0.769, then to coughing, 0.958, are high, and these observations indicate that a big portion of infected symptomatic patients may follow this path. Finally, this implementation of the model predicts that nausea/vomiting occurs earlier diarrhea. These 2 results advise that in patients with SARS-CoV-2, the body start develops fever, and then upper respiratory symptoms and finally symptoms of the upper then lower gastrointestinal (GI) tract.
To farther investigate these symptom paths, nosotros implemented the Stochastic Progression Model with the main dataset (COVID-19 with N = 55,924) (viii), to decide the probable downstream paths when the get-go one or two symptoms are forced to a certain state (Figures 1F–I). The gray lines represent the "forced" paths. The rest of the paths were determined equally before with a greedy algorithmic approach. Nosotros constitute that the most likely orders of the downstream path are consistent with the nigh probable orders of the unforced paths. Even if the kickoff symptom is forced to be an unlikely one (due east.thou., diarrhea), the downstream paths maintain the most likely order of the other three symptoms that we originally determined (Figure 1F). Similarly, the GI tract effects occur outset in the forced to the lowest degree likely paths (Figure 1G). When forcing the path one step further by predetermining the first 2 symptoms for both the most and to the lowest degree likely paths, the findings remain the aforementioned (Figures 1H,I).
Order of Discernible Symptoms in COVID-nineteen Is Independent of Severity of Disease on Admission
The confirmation dataset of COVID-xix cases (N = one,099) separates the reported ane,099 cases between severe and non-astringent patients every bit designated on admission (16). To investigate the effects of severity on the order of discernible symptoms, we implemented each ready of cases separately using the Stochastic Progression Model. We found that the most and to the lowest degree probable paths are identical in severe and non-severe cases and to our original findings higher up (Figure two). To illustrate the similarities, the largest divergence in likelihood is observed when transitioning from no symptoms to fever in the most likely path. In severe and non-severe cases, the probability is 0.775 and 0.818, respectively, indicating a divergence of 0.043. These results suggest that severity does not impact the order of discernible symptoms, and they are consequent with the hypothesis of fever every bit the outset symptom of COVID-19.
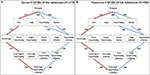
Figure 2. The most and least probable paths of discernible symptoms in severe and non-astringent COVID-19 cases on admission. (A) Hasse Diagram of the most likely paths (red) and least likely paths (bluish) in COVID-19 for cases designated as severe on admission determined from transition probabilities presented here. (B) Hasse Diagram of the most likely paths (ruby-red) and least likely paths (blue) in COVID-19 for cases designated as not-severe on access determined from transition probabilities presented here.
Variation of Club of Discernible Symptoms Betwixt Respiratory Diseases
The four discernible symptoms are objective and relatively easy for patients and clinicians to ostend. So, we developed implementations of the Stochastic Progression Model using these symptoms to determine the virtually likely and least probable paths for 4 respiratory diseases: COVID-19, influenza, MERS, and SARS (Figures 3A–D) (six–8). The most likely gild of occurrence of symptoms in COVID-19 is fever, coughing, nausea/airsickness, and diarrhea (Figure 3A). This path is identical to flu except the order of the initial two symptoms is switched (Effigy 3B). On the other hand, the predicted most likely paths (i.due east., fever, coughing, diarrhea, and and then nausea/vomiting) are the aforementioned for MERS and SARS (Figures 3C,D). This society has ane difference from the well-nigh likely path in COVID-19 in that the club of the concluding ii symptoms are reversed. The least likely path of MERS starts with either nausea/vomiting or diarrhea as the first step. These steps are followed by coughing, and finally fever. In contrast, the least likely path of SARS is cough, nausea/airsickness, and diarrhea in any gild, and and so finally fever. Notwithstanding, the to the lowest degree probable path of symptoms in COVID-19 is the same as the least likely path in MERS, and the to the lowest degree likely path of influenza is unique compared to the other diseases. It is not detectable whether nausea/airsickness or diarrhea are the beginning symptoms in influenza, but afterwards these 2, the least likely path continues from there to fever then cough. This ascertainment further illustrates the strong link of cough to flu. As for coronavirus-related diseases, the strongest showtime indicator is fever followed past coughing.
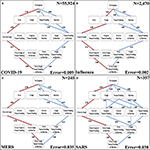
Figure 3. The most likely and least likely paths of discernible symptoms in respiratory diseases. (A) The most likely paths (red) and to the lowest degree likely paths (blue) in a Hasse Diagram for COVID-19 symptoms. (B) The most probable paths (red) and to the lowest degree probable paths (blue) in a Hasse Diagram for influenza symptoms. (C) The nearly likely paths (red) and least probable paths (blueish) in a Hasse Diagram for MERS symptoms. (D) The most likely paths (red) and to the lowest degree likely paths (blue) in a Hasse Diagram for SARS symptoms. For each diagram, the most and least likely paths are determined from the transition probabilities that are depicted on the edges. Additionally, fault of transition probabilities and sample size (Due north) are presented.
Comparing the Order of About Mutual Symptoms in Respiratory Diseases With COVID-19
Although active surveillance of the order discernible symptoms (i.e., fever, coughing, nausea/vomiting, and diarrhea) could exist useful due to the distinctive almost and to the lowest degree probable paths that we determined, nosotros expanded our assay to the seven symptoms commonly observed in all iv respiratory diseases studied here. So, we created a second set of symptoms that apology sore throat, myalgia, and headache to the original fix of symptoms (Supplemental Table 2). The three additional symptoms are more subjective (half dozen–8). The seven-symptom implementation of the Stochastic Progression Model of COVID-19 shows that these additional symptoms did non perturb our initial ordering of fever, coughing, nausea/airsickness, and diarrhea, but instead added another level of intricacy in the middle of the likely paths (Figure 4). Nosotros still find that the most likely path first transitions to fever, indicating that fever is the most likely start symptom. From in that location, the almost likely next symptom is coughing once more. So, we find an undetectable difference in likelihood of transitioning to either sore pharynx, headache, or myalgia, indicating that all three are likely to occur next before proceeding. The final two nodes are consistent with the 4-symptom order past indicating that nausea/vomiting then diarrhea occur last. Although this implementation is more complex because information technology has seven symptoms, information technology is consequent with our earlier findings. The virtually probable path of COVID-nineteen symptoms is fever, then cough, and next either sore pharynx, myalgia, or headache, followed past nausea/airsickness, and finally diarrhea, and this order is the same as the one indicated by the implementation developed from the confirmation dataset (COVID-19 with Northward = 1,099) (Figure 4) (16).
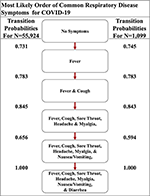
Figure iv. The nigh likely path of common respiratory symptoms in COVID-19. The most likely path of 7 common symptoms of COVID-19, determined past the transition probabilities that are also listed betwixt nodes, of two datasets here.
We also implemented the Stochastic Progression Model with the aforementioned seven symptoms in influenza, SARS, and MERS datasets to compare and contrast illness progression with that in COVID-19 (Effigy 5) (6–eight). The results for flu indicate that cough or myalgia may occur first (Effigy 5A). After these two symptoms occur, the order of symptoms is headache, sore throat and fever. Finally, airsickness/nausea and diarrhea have an undetectable difference in probability of occurring last. The MERS implementation displays a almost likely path in which fever will occur showtime, followed by cough, headache, and then myalgia (Effigy 5B). These are followed by an undetectable difference in likelihood of headache and diarrhea occurring. Finally, sore pharynx and nausea/vomiting will occur concluding with an undetectable difference. The implementation for SARS shows that fever is most likely to occur first, followed by an undetectable difference in transition probability of cough and myalgia, which is similar to the other coronavirus-related diseases (Figure 5C). Next, headache is most probable. Finally, diarrhea, sore pharynx and nausea/vomiting occur with an undetectable difference in likelihood.
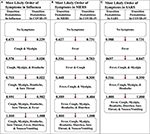
Figure 5. The most likely paths of symptoms in flu, MERS, and SARS vs. COVID-xix. (A) The most likely path of seven common symptoms of influenza with the transition probabilities listed between nodes. (B) The most likely path of seven common symptoms of MERS with the transition probabilities listed between nodes. (C) The most likely path of 7 common symptoms of SARS with the transition probabilities listed between nodes. For each path, the transition probabilities in COVID-19 are listed on the correct. The most likely paths for each respective illness hither are determined from the transition probabilities listed betwixt nodes on the left.
To illustrate the uniqueness of the most likely path of COVID-19, we found the transition probabilities of the aforementioned path in the other respiratory diseases (Figure vi). When comparison and contrasting the probabilities, we found that the implementation representing COVID-nineteen strongly asserts that the start symptom volition be fever and cough will soon follow considering the transition probabilities are 0.731 and 0.783, respectively (Effigy 6A), whereas the flu implementation indicates that fever is very unlikely to occur starting time with a probability of but 0.035 (Effigy 6B). Additionally, the implementations of MERS and SARS data also take a loftier likelihood of transitioning to fever first, with a probability of 0.627 and 0.988, respectively (Figures 6C,D). The second symptom of the most likely path of COVID-19 is cough, with a probability of 0.783, but the others practice not have a similar loftier probability. For example, the respiratory affliction with the highest probability at that transition is MERS at 0.536. Still, after fever and cough, COVID-nineteen and the other three respiratory diseases accept a similarly high likelihood of the three subjective symptoms (i.e., sore throat, headache, and myalgia). Finally, the virtually probable path of COVID-xix ends with nausea/vomiting and so diarrhea. These observations are consistent with the symptoms described by the CDC and support the notion that fever followed by cough seems highly probable to be diagnosed as COVID-19 (five).
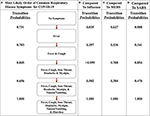
Effigy vi. The almost likely path of symptoms in COVID-19 vs. flu, MERS, and SARS. (A) The most likely path of 7 common symptoms of COVID-xix with the transition probabilities listed between nodes. (B) The transition probabilities of the path of influenza. (C) The transition probabilities of the path of MERS. (D) The transition probabilities of the path of SARS. The most likely path here is determined from the transition probabilities listed between nodes for COVID-19.
Also, comparing the transition probabilities of paths in the aforementioned affliction illustrates the significance of the most likely pathways. For example, the lowest transition probability in the most probable path of flu is 0.578 (Effigy 5A), whereas when analyzing influenza every bit it traverses down the most likely path of COVID-nineteen, the transition probabilities observed are 0.v or less (Figure 6B). Nonetheless, in that same path, the transition probability from fever and cough to fever, cough, sore throat, headache, and myalgia is >0.999. This value displays how unlikely nausea/vomiting and diarrhea are to be initial symptoms of influenza. Additionally, when observing the most probable path of COVID-19, the kickoff two symptoms seem to have a potent probability of occurring in the society of fever and then cough, with a likelihood of 0.731 (Figure 5A). All the same, the likelihood of cough occurring first in COVID-19 is 0.229, which is a depression probability (Figure 5A). This ascertainment further supports the hypothesis of fever occurring beginning and coughing occurring second.
Call back and Selectivity When Linking First Symptom and Disease
The COVID-xix and influenza implementations of the Stochastic Progression Model suggest that there is a high likelihood of fever and coughing occurring get-go, respectively. Nosotros desired to observe metrics quantifying the possible link between first symptom and these two diseases. And then, we adamant the recall and the selectivity when using the initial symptom as an indicator of COVID-19 or flu, with all other possible diseases excluded in a theoretical patient population. First, we imitation patient datasets using reported data that were independent from all previous work that nosotros integrated in our analyses above (Supplemental Tabular array v) (20). Ii simulated patient datasets were created to analyze COVID-nineteen and influenza separately to portray the specific link of each illness with the corresponding initial symptom that we adamant, fever and cough, respectively. The faux data contained data about the patients' state of affliction (COVID-xix, influenza or not) and their starting time symptom experienced. Based on the information of the first symptom alone, nosotros categorized the fake patient information as infected with COVID-xix or not and flu or not. The recall was calculated equally the number of fake patients that nosotros correctly identified as having the illness over the number of simulated patients that truly had the disease (27). Selectivity was defined hither as the number of simulated patients that nosotros correctly identified every bit non having the disease over the number of simulated patients that truly did non have the illness (28). For both diseases, nosotros performed this assay for v fake samples of unlike sizes, each containing 5% infected individuals. We repeated this procedure 10 times and calculated the boilerplate and standard deviation across each sample size for both COVID-19 and influenza (Tables 1, 2).
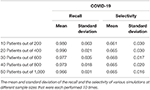
Tabular array 1. Call up and selectivity of linking fever as a first symptom of patients with COVID-19.
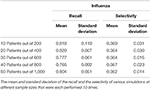
Tabular array ii. Recall and selectivity of linking cough as a first symptom of patients with flu.
The call up ranges from 0.966 to 0.990 with a standard divergence of 0.031 and 0.021, respectively when analyzing the link betwixt COVID-19 and fever as a first symptom. The maximum standard divergence of whatever sample size is 0.063 for the mean of 0.980. On the other manus, the selectivity of fever as a first symptom of COVID-xix ranges from 0.661 to 0.668 with a standard deviation of 0.030 and 0.020, respectively, and 0.030 is the maximum standard deviation with corresponding means of 0.661 and 0.665 (Tabular array 1). Equally for coughing every bit a first symptom of influenza, the remember ranges from 0.765 to 0.820 with corresponding standard deviations 0.092 and 0.067. The highest standard divergence is 0.110 with a mean of 0.810, and the selectivity ranges from 0.362 to 0.369 with standard deviations of 0.014 and 0.031, respectively, and the maximum standard departure is 0.031 (Tabular array 2).
The recall in both cases is lower than the selectivity, and this observation indicates that this analysis categorizes patients equally infected when they are not, just the high recollect indicates that nigh infected patients did align with the first symptom that nosotros predicted. In the future, we expect to confirm this assay with information on first symptoms, as opposed to simulated information, simply the purpose of this analysis was to brandish that farther written report of club of symptoms might lead to earlier recognition.
Discussion
In this study, we found evidence that supports the notion that there is a near common club of discernible symptoms in COVID-nineteen that is also different from other prominent respiratory diseases. The near likely initial symptom is fever in the iii diseases studied that are acquired past coronaviruses (i.e., COVID-19, SARS, and MERS) and cough in influenza. The most likely order of the four hands discernible symptoms is identical in MERS and SARS, but the nigh probable path of COVID-xix has one cardinal difference. The offset two symptoms of COVID-nineteen, SARS, and MERS are fever and coughing. However, the upper GI tract (i.e., nausea/airsickness) seems to be affected before the lower GI tract (i.east., diarrhea) in COVID-19, which is the opposite from MERS and SARS. In all diseases, we institute that fever and cough occur before nausea/airsickness and diarrhea. When observing the fix of 7 symptoms including three subjective ones (i.eastward., sore throat, headache, and myalgia), we found that the initial symptoms of the most likely path are the aforementioned as in the most likely path of the four discernible symptoms. Also, in both the 4 and seven symptoms implementations, the GI tract symptoms are last. A dissever MERS dataset included the initial symptoms of patients on admission, which listed the symptoms from highest to lowest probability as fever, myalgia, coughing, and diarrhea (18). This order is like to the about likely path that we adamant. A very small percent of patients experienced diarrhea as an initial symptom. This report suggests that diarrhea as an early symptom indicates a more ambitious disease, because each patient in this dataset that initially experienced diarrhea had pneumonia or respiratory failure somewhen (Supplemental Table three). We advise that these patients may be experiencing a more aggressive grade of the disease and have accelerated through the nearly likely path, having already experienced diarrhea. These findings marshal with another dataset provided for SARS, which also contained the percentage of the various symptoms to be reported first (Supplemental Table 4). The highest reported symptom is fever, followed by cough or dyspnea, and then finally, a pocket-size percent of patients reported diarrhea (19). This order confirms the most likely paths that we have determined. The observation that diarrhea was very uncommon as a first symptom and had a not-nil probability of occurrence is consequent with our assay. This aligns with our hypothesis that early occurrence of diarrhea implies that those patients may have a much more than aggressive form of the disease.
The simulation data used to gauge the land and transition probabilities in the Stochastic Progression Model relies on the assumption that symptoms included in the model are independent. Using the definition of independence, we observed the individual probabilities of fever and cough in a dataset from a example study of influenza, and we constitute that the production of the individual probabilities of fever and cough is nearly equal to the probability of both occurring (21). Considering this outcome, we proceeded under the assumption of independence, which we volition reevaluate when more symptom data becomes available. We simulated combinations of symptoms for 500,000 patients, which we chose considering information technology was the everyman attempted number that empirically produced the theoretical expected effect for random frequency symptoms: that all paths would be every bit likely, up to 100ths of a decimal place. We and then utilized these simulated patients to approximate the country probabilities and transition probabilities described above.
This report supports the idea that symptoms occur in a predictable club, simply future work is needed to amend aspects of the Stochastic Progression Model and ostend the results plant here. Our finding that COVID-19 start presents with a fever supports the recommended measures by the CDC which state that the public should take their temperature at habitation and when entering facilities every bit an early on checking method (29). This application of the Stochastic Progression Model may be improved if at that place were objective ways to measure the more subjective symptoms (i.e., sore throat, headache, and myalgia). Also, improved error calculations of the transition probabilities would lead to more accurate results. Our current error calculation is conservative, because when more symptoms were added, we observed that the error compounded as we progressed further down the paths (Supplemental Figures 2–13). The bourgeois error estimate creates bug in discerning the difference in probabilities of symptoms. Specifically, in implementations of seven symptoms, the likelihoods are more difficult to ascertain due to subjective reporting and compounding error calculations. Datasets that incorporate the society of symptoms for each patient would lower the error. Additionally, these sorts of datasets would meliorate the approximations of the transition probabilities and increase accurateness. This improvement could be achieved by physicians implementing the exercise of recording the order of occurrence of symptoms. With this information, we may gauge the likelihood of a patient acquiring a symptom based on their current symptoms with patient data instead of simulations based on frequency. Applying objective criteria for symptoms, improving error calculations, and collecting the order of symptoms would non only permit united states to improve our findings hither, but also allow the Stochastic Progression Model to predict orders of a larger set up of symptoms. The optimal class of the Stochastic Progression Model would be adult by determining state probabilities from observed true frequencies of patients' symptoms and determining transition probabilities from the patients' true order of symptoms. However, until this data is bachelor, improved approximations, simulations and error calculations are needed.
Furthermore, when analyzing fever equally the first symptom of COVID-19, a depression selectivity indicates a loftier Type I error (i.eastward., rate of fake positive), and a high call back indicates a depression Type Ii error (i.east., rate of fake negative). We found a moderate selectivity value and as a effect, a moderate Type I error in this example. This Blazon I mistake is acceptable in our use of investigating fever every bit an initial symptom of COVID-19, because information technology suggests that more people become tested who are not infected, rather than less people become tested who are infected, as with Type Ii mistake (thirty). We are not proposing initial symptom as a diagnostic examination, but instead as a possible sign to get tested. COVID-xix outbreaks in clusters, and these unusual clusters of disease are characteristic of a pandemic disease that must exist addressed immediately with aggressive testing to curb transmission (31).
The importance of knowing starting time symptoms is rooted in the need to cease the spread of COVID-nineteen, a illness that is two to three times more transmissible than influenza and results in outbreaks of clusters (iii, 4). There is a heightened risk in COVID-19 existence passed on, so faster testing and social distancing are of import, especially when social distancing and quarantine measures are relaxed. Our results assert that fever is the about likely symptom to occur first in symptomatic adult patients with COVID-xix. Nosotros promise that the hypotheses generated in this work are tested with prospective clinical information to confirm that a coughing occurs first more frequently in influenza and also fever in COVID-19. We believe that early on detectors that any individual tin can recognize to seek medical attention before is useful. In addition, datasets that contain information of symptom order and strains of COVID-19 allow for further studies that may make up one's mind whether onset of symptoms vary in specific strains (32), and whether risk factors, such every bit obesity (33), and ecology factors, such as temperature (34) impact symptom order. To boring the spread of COVID-xix, our results back up the practise that fever should be tested before assuasive entry to facilities and that those with fever should immediately seek medical attention for diagnosis and contact tracing. Such measures as these may help to reduce transmission despite the high contamination of SARS-CoV-2.
Information Availability Statement
Publicly available datasets were analyzed for this report. These tin exist constitute here: https://www.who.int/publications-detail/report-of-the-who-people's republic of china-joint-mission-on-coronavirus- disease-2019-(covid-19), https://world wide web.nejm.org/doi/total/x.1056/NEJMoa2002032, https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/485554, https://onlinelibrary.wiley.com/doi/full/x.1111/resp.13196, https://www.journalofinfection.com/article/S0163-44531630209-2/abstruse, https://jamanetwork.com/journals/jama/fullarticle/196681, https://jamanetwork.com/journals/jama/fullarticle/2761044, https://world wide web.cambridge.org/cadre/journals/infection-control-and-infirmary-epidemio logy/article/symptoms-of-flu-virus-infection-in-hospitalized-patients/8F1B478BA4B861D356393EA77AD8B83B#.
Author Contributions
JL and JH conceived the model. JL and JM conceived the project. JL created the model. JL, MM, and JM analyzed results. JL and MM wrote the manuscript. PK and JH supervised the project. All authors read, edited, and canonical the concluding manuscript.
Funding
This work was supported by the National Cancer Institute (Award Number U54CA143906 and P30CA014089) and the Carol Vassiliadis fellowship. JL was supported by the USC Dana and David Dornsife College of Letters, Arts and Sciences, and the Schlegel Family Endowment Fellowship.
Disharmonize of Involvement
MM is employed by the company Nexus Development PA LLC. JM is employed by the company NanoCarrier Co., Ltd.
The remaining authors declare that the inquiry was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Nosotros wish to thank Dr. Jorge Nieva for discussions and advisement and Libere Ndacayisaba for critical reading of the manuscript.
Supplementary Material
The Supplementary Cloth for this article can be found online at: https://www.frontiersin.org/manufactures/10.3389/fpubh.2020.00473/total#supplementary-material
References
2. Cascella K, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19). StatPearls Publishing (2020).
Google Scholar
3. Anderson RM, Heesterbeek H, Klinkenberg D, Hollingsworth TD. How will country-based mitigation measures influence the course of the COVID-xix epidemic? Lancet. (2020) 395:931–4. doi: ten.1016/S0140-6736(20)30567-5
PubMed Abstract | CrossRef Full Text | Google Scholar
four. Lord's day K, Chen J, Viboud C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced information: a population-level observational study. Lancet Digital Health. (2020) 2:E201–8. doi: ten.1016/S2589-7500(20)30026-1
PubMed Abstract | CrossRef Full Text | Google Scholar
five. Sohrabi C, Alsafi Z, O'Neill N, Khan M, Kerwan A, Al-Jabir A, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-nineteen). Int J Surg. (2020) 76:71–half-dozen. doi: 10.1016/j.ijsu.2020.02.034
PubMed Abstruse | CrossRef Full Text | Google Scholar
6. Monto As, Gravenstein S, Elliott Thousand, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Archiv Internal Med. (2000) 160:3243–7. doi: 10.1001/archinte.160.21.3243
PubMed Abstruse | CrossRef Full Text | Google Scholar
nine. Reed C, Kim IK, Singleton JA, Chaves SS, Flannery B, Finelli L, et al. Estimated influenza illnesses and hospitalizations averted by vaccination—United states of america, 2013–14 influenza season. MMWR Morbidity bloodshed Weekly Report. (2014) 63:1151–4.
PubMed Abstruse
10. Newton PK, Bricklayer J, Bethel Thousand, Bazhenova LA, Nieva J, Kuhn P. A stochastic Markov chain model to describe lung cancer growth and metastasis. PLoS ONE. (2012) 7:34367. doi: 10.1371/journal.pone.0034637
PubMed Abstract | CrossRef Full Text | Google Scholar
xi. Newton PK, Mason J, Bethel K, Bazhenova 50, Nieva J, Norton L, et al. Spreaders and sponges define metastasis in lung cancer: a Markoff chain Monte Carlo mathematical model. Cancer Res. (2013) 73:2760–nine. doi: 10.1158/0008-5472.CAN-12-4488
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Bazhenova L, Newton P, Mason J, Bethel K, Nieva J, Kuhn P. Adrenal metastases in lung cancer: clinical implications of a mathematical model. J Thoracic Oncol. (2014) 9:442–half-dozen. doi: 10.1097/JTO.0000000000000133
PubMed Abstract | CrossRef Total Text | Google Scholar
xiii. Newton PK, Bricklayer J, Hurt B, Bethel Thou, Bazhenova L, Nieva J, et al. Entropy, complexity, and Markov diagrams for random walk cancer models. Sci Rep. (2014) 4:7558. doi: 10.1038/srep07558
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Newton PK, Bricklayer J, Venkatappa Northward, Jochelson MS, Hurt B, Nieva J, et al. Spatiotemporal progression of metastatic chest cancer: a Markov chain model highlighting the part of early metastatic sites. NPJ Breast Cancer. (2015) 1:ane–9. doi: 10.1038/npjbcancer.2015.eighteen
PubMed Abstract | CrossRef Total Text | Google Scholar
15. Eriksson K, Jonsson Thousand, Sjöstrand J. Markov chains on graded posets. Order. (2018) 35:93–109. doi: 10.1007/s11083-016-9420-one
CrossRef Full Text | Google Scholar
16. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus illness 2019 in Red china. N Engl J Med. (2020) 382:1708–20. doi: x.1056/NEJMoa2002032
CrossRef Full Text | Google Scholar
17. Chan MC, Lee N, Chan PK, To Thousand, Wong RY, Ho W-Southward, et al. Seasonal influenza A virus in feces of hospitalized adults. Emerg Infectious Dis. (2011) 17:2038. doi: 10.3201/eid1711.110205
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Ko J-H, Park GE, Lee JY, Lee JY, Cho SY, Ha YE, et al. Predictive factors for pneumonia evolution and progression to respiratory failure in MERS-CoV infected patients. J Infection. (2016) 73:468–75. doi: 10.1016/j.jinf.2016.08.005
PubMed Abstruse | CrossRef Full Text | Google Scholar
nineteen. Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, et al. Clinical features and brusque-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. (2003) 289:2801–ix. doi: 10.1001/jama.289.21.JOC30885
PubMed Abstract | CrossRef Total Text | Google Scholar
twenty. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Van den Dool C, Hak E, Wallinga J, Van Loon A, Lammers J, Bonten K. Symptoms of influenza virus infection in hospitalized patients. Infection Control Hospital Epidemiol. (2008) 29:314–9. doi: 10.1086/529211
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. (2018). Available online at: https://www.R-project.org/ (accessed February 13, 2019).
Google Scholar
24. Varga Z, Flammer AJ, Steiger P, Haberecker Yard, Andermatt R, Zinkernagel As, et al. Endothelial jail cell infection and endotheliitis in COVID-xix. Lancet. (2020) 395:1417–8. doi: 10.1016/S0140-6736(20)30937-5
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement subsequently infection with COVID-19 and other coronaviruses. Brain Behavior Immunity. (2020) 87:18–22. doi: 10.1016/j.bbi.2020.03.031
PubMed Abstruse | CrossRef Full Text | Google Scholar
26. Diaz-Guimaraens B, Dominguez-Santas Chiliad, Suarez-Valle A, Pindado-Ortega C, Selda-Enriquez Thousand, Bea-Ardebol S, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus ii infection. JAMA Dermatol. (2020) 156:820–two. doi: 10.1001/jamadermatol.2020.1741
PubMed Abstract | CrossRef Total Text | Google Scholar
27. Powers DMW. Evaluation: from precision, call up and fmeasure to ROC, informedness, markedness and correlation. J Mach Acquire Technol. two:37–63.
Google Scholar
29. Ng Y, Li Z, Chua YX, Chaw WL, Zhao Z, Er B, et al. Evaluation of the effectiveness of surveillance and containment measures for the starting time 100 patients with COVID-19 in Singapore–January 2–February 29, 2020. Centers Dis Control Prev. (2020) 69:307–eleven. doi: 10.15585/mmwr.mm6911e1
PubMed Abstruse | CrossRef Full Text | Google Scholar
30. Pericchi 50, Pereira C. Adaptative significance levels using optimal decision rules: balancing by weighting the error probabilities. Brazil J Probabil Statist. (2016) 30:70–90. doi: 10.1214/fourteen-BJPS257
CrossRef Full Text | Google Scholar
31. Hitchcock P, Chamberlain A, Van Wagoner M, Inglesby TV, O'Toole T. Challenges to global surveillance and response to communicable diseases outbreaks of international importance. Biosecurity Bioterrorism. (2007) 5:206–27. doi: 10.1089/bsp.2007.0041
PubMed Abstract | CrossRef Total Text | Google Scholar
32. Tang X, Wu C, Li 10, Song Y, Yao X, Wu X, et al. On the origin and continuing evolution of SARS-CoV-two. Natl Sci Rev. (2020) seven:nwaa036. doi: x.1093/nsr/nwaa036
CrossRef Full Text | Google Scholar
33. Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk cistron for Covid-19 hospital admission. Clin Infect Dis. (2020) 71:ciaa415. doi: 10.1093/cid/ciaa415
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fpubh.2020.00473/full
0 Response to "Which of the Following Is an Effective Model for Understanding the Role of Symptoms in the Family?"
Postar um comentário